Upload Manufacturer Instrument File
Manufacturers have two ways of uploading data into the ![]() 340B OPAIS The 340B Office of Pharmacy Affairs Information System (OPAIS) is a collection of information submitted by covered entities, contract pharmacies, and manufacturers maintained and verified by HRSA's Office of Pharmacy Affairs (OPA).. Data can be uploaded as a fixed length .txt instrument file or the data can be entered manually. If a manufacturer decides to upload their data as a fixed length .txt instrument file, it must be in the .txt file format identified in the chart below.
340B OPAIS The 340B Office of Pharmacy Affairs Information System (OPAIS) is a collection of information submitted by covered entities, contract pharmacies, and manufacturers maintained and verified by HRSA's Office of Pharmacy Affairs (OPA).. Data can be uploaded as a fixed length .txt instrument file or the data can be entered manually. If a manufacturer decides to upload their data as a fixed length .txt instrument file, it must be in the .txt file format identified in the chart below.
-
Select Manufacturer Quarterly Upload then Upload Manufacturer Instrument File to upload your quarterly pricing data during the manufacturer submission period. A manufacturer can upload multiple files multiple times during the quarterly submission period. The latest uploaded data for a specific NDC will override the previous data for a specific NDC and reconciliation tasks will be created based on the latest data uploaded for a specific NDC.
-
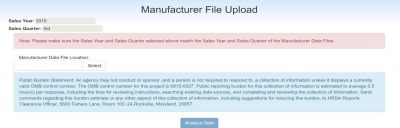
The Manufacturer File Upload page is displayed below.
-
Select the Sales Year and Sales Quarter to match the year and quarter of the data upload file.
-
Click the blank box or the Select button to locate and open the instrument file.
-
Click the Select button.
-
Selecting the file will start the upload immediately.
-
The file will appear on the Manufacturer File Upload page with a Remove link to allow you to remove it and select a different file.
-
The Analyze Data button will be enabled.
-
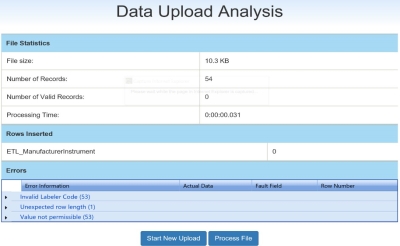
The Data Upload Analysis page displays the file statistics for the upload, the number of rows inserted into the database, and the number and type of .txt file errors. Rows with identified errors will not be processed. There are different options to address file errors before selecting Process File.
-
Create a new instrument file with the identified errors corrected and upload it. The 340B OPAIS accepts the most recently uploaded data for each NDC. In other words, the 340B OPAIS will overwrite any previously submitted data for an NDC.
-
Create a new instrument file that contains all required fields for each NDC that had an identified error in the previous upload. Upload this second instrument file and the 340B OPAIS will accept the most recently uploaded data for each NDC in the file. Previously submitted data for different rows/NDCs will not be deleted or overwritten in the 340B OPAIS.
-
Manually enter data for each NDC that had an identified error.
-
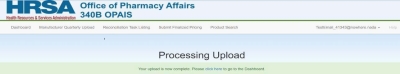
Upon clicking the Process File button, the system processes and loads the records, and displays the Processing Upload page.
-
Return to the dashboard to view the quarterly submission progress status.
Click the small arrows to expand/collapse the list to display error details. The table below displays commonly encountered error messages.
| Error Message | Description |
|---|---|
| "This combination of NDC, SalesYear and
SalesQuarter was not found in the |
The drug code is not present in the FDB file for the specified sales quarter and sales year. |
| "Supplied Ceiling Price does not match Calculated Ceiling Price" | There is a mismatch between the ceiling price in the Instrument file and the ceiling price calculated by OPAIS using the AMP and URA in the file. |
| "This Labeler Code is not allowed for this user" | Manufacturer is not allowed to submit the prices for this Labeler Code. |
| "NDC is not available for reporting this period.” | Manufacturer is not allowed to submit the prices for this drug code. |